Thermodynamics is a branch of physics that deals with the study of energy and its transformation from one form to another. It encompasses the relationships between heat, temperature, work, and energy, and how they relate to each other. The principles of thermodynamics have broad applications in physics, chemistry, engineering, and many other fields.
The field of thermodynamics
The field of thermodynamics is built upon a set of fundamental laws that govern the behavior of systems in thermodynamic equilibrium. These laws are the first law of thermodynamics, the second law of thermodynamics, and the third law of thermodynamics.
Thermodynamics also includes the concept of entropy, which is a measure of the disorder or randomness of a system. The second law of thermodynamics states that the total entropy of a closed system can never decrease, which means that the disorder of a system will always tend to increase over time. This concept is crucial for understanding why certain processes are irreversible, such as the expansion of a gas into a vacuum.
Applications of thermodynamics
Applications of thermodynamics are wide-ranging and have significant impacts on our daily lives. For example, thermodynamics is used in the design of engines, refrigeration systems, and power plants. It is also essential in understanding the behavior of materials at high and low temperatures, such as in the production of ceramics, superconductors, and semiconductors. In the field of biology, thermodynamics is used to understand the behavior of biological systems and the transfer of energy in biological processes.
thermodynamics is a fascinating field that helps us understand the behavior of energy in various systems. It provides a foundation for many critical applications in science and technology and has broad applications in many different fields. Understanding the principles of thermodynamics is essential for engineers, scientists, and anyone interested in energy and its transformation.
Engineering Thermodynamics Explained
Thermodynamics is the branch of physics that deals with the study of heat and temperature and their relation to energy and work. Engineering thermodynamics, on the other hand, applies the principles of thermodynamics to engineering systems and processes. In this blog, we will delve deeper into the world of engineering thermodynamics and explore its applications, laws, and concepts.
Applications of Engineering Thermodynamics
Engineering thermodynamics has numerous applications in various fields, including mechanical engineering, chemical engineering, aerospace engineering, and energy systems engineering, among others. Some of the common applications of engineering thermodynamics include:
Heat engines: Heat engines are devices that convert thermal energy into mechanical energy. They are used in various applications, including automobiles, power plants, and aircraft engines.
Refrigeration and air conditioning: Thermodynamics principles are applied to refrigeration and air conditioning systems to cool down an enclosed space or substance.
Power generation: Thermodynamics is used in power generation systems, such as gas turbines and steam turbines, to convert thermal energy into electrical energy.
Combustion engines: Combustion engines, such as internal combustion engines, use thermodynamics principles to convert the energy released by burning fuel into mechanical energy.
Laws of Thermodynamics
The laws of thermodynamics are fundamental principles that govern the behavior of energy and matter in a system. They form the basis of engineering thermodynamics and are essential in understanding how energy is transformed in different systems. The laws of thermodynamics are as follows:
First law of thermodynamics:-
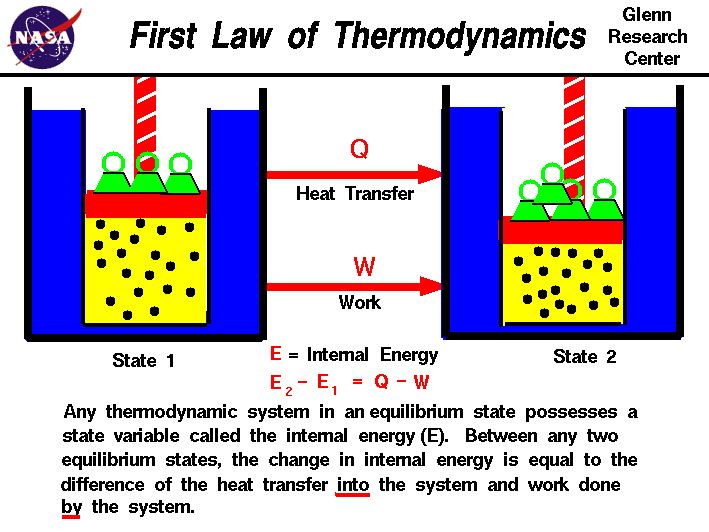
The first law of thermodynamics is also known as the law of conservation of energy. It states that energy cannot be created or destroyed, only transferred or converted from one form to another. This law forms the basis for understanding how energy is conserved in various thermodynamic processes, such as heating and cooling systems, and power generation.
Simpaly, This law states that energy cannot be created or destroyed, but it can be converted from one form to another. In other words, the total energy in a closed system remains constant.
Second law of thermodynamics:-
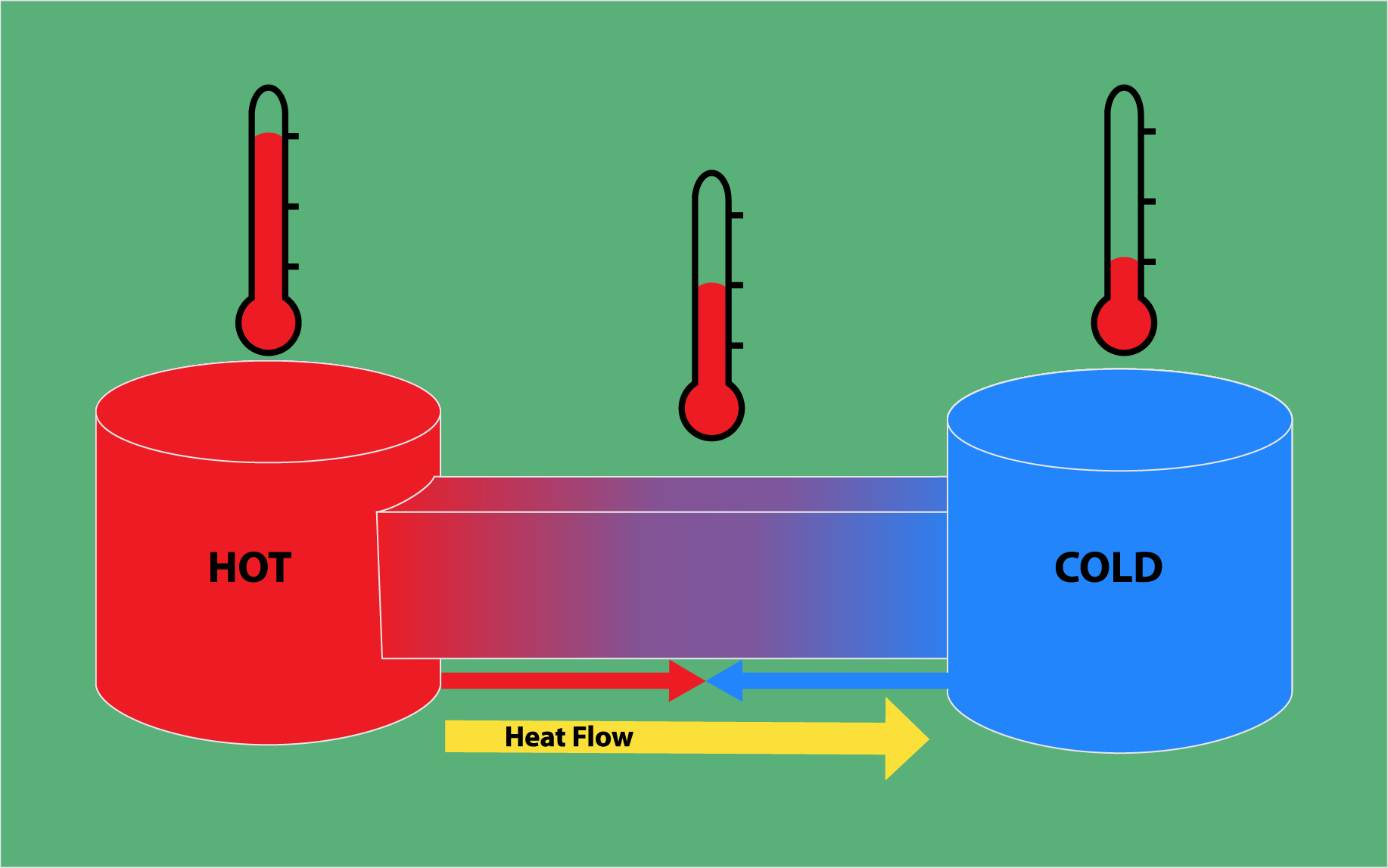
The second law of thermodynamics is concerned with the direction of heat flow and the efficiency of energy transfer. It states that heat always flows from a hotter object to a colder object and that the efficiency of energy transfer is always less than 100%. This law is critical to understanding why energy conversion processes are never 100% efficient, and why there is always some energy lost as waste heat.
This law states that the total entropy of a closed system always increases over time. Entropy is a measure of the disorder or randomness in a system.
Third law of thermodynamics:-
The third law of thermodynamics deals with the behavior of systems as they approach absolute zero temperature. It states that it is impossible to reach absolute zero temperature through any finite number of steps. This law is critical to understanding the behavior of materials at extremely low temperatures, such as in superconductors and superfluids.
This law states that it is impossible to reach absolute zero temperature (0 Kelvin or -273.15°C) through any finite number of processes.
Concepts in Engineering Thermodynamics
Engineering thermodynamics involves several key concepts that are important in understanding the behavior of energy in various systems. Some of the essential concepts in engineering thermodynamics include:
Enthalpy: Enthalpy is a measure of the energy of a system and is defined as the sum of the internal energy and the product of pressure and volume.
Entropy: Entropy is a measure of the disorder or randomness in a system. It increases over time in a closed system, as per the second law of thermodynamics.
Heat capacity: Heat capacity is the amount of heat required to raise the temperature of a substance by a certain amount. It is measured in Joules per Kelvin (J/K).
Heat transfer: Heat transfer refers to the movement of thermal energy from one system to another due to a temperature difference. It can occur through conduction, convection, and radiation.
Conclusion
Engineering thermodynamics is a vital field that applies the principles of thermodynamics to various engineering systems and processes. It has numerous applications in different fields, including mechanical engineering, chemical engineering, aerospace engineering, and energy systems engineering. Understanding the laws and concepts of thermodynamics is essential in designing efficient and sustainable energy systems.